Client story Global Clinical Trials Kept on Track with Device Lifecycle Management

By Insight UK / 5 Jul 2021
An international clinical trials company, which offers a specialist software platform to leading pharmaceutical companies enabling clinical trials and data collection, wanted to expand its business.
The client needed to ship digital devices to medical experts around the world, to enable them to enter trial data onto its digital platform. The sensitive patient data would then need to be removed from the devices to comply with industry regulations.
Insight proposed a complete device lifecycle management solution to help the client scale up its services to meet growing demand.
The Challenge
Clinical drug trials are prone to high failure rates due to the subjective nature of ratings, leading to bias, variability and errors.
To overcome these problems, the client developed a digital platform that enables specially selected medical experts to assess and report on clinical outcomes remotely.
Clinicians visit the patients involved in drug trials and enter data on their treatment outcomes into the platform tablet devices. This data is then shared, centralised and analysed on a web-based portal.
The digital platform has proved so successful that the client wanted to expand the solution to customers around the world.
To achieve this, the company needed an IT infrastructure capable of large-scale delivery while complying with strict pharmaceutical industry procedures.
The Solution
The client needed to focus on its core business of developing cutting edge clinical trial solutions, so it turned to Insight to take care of its service delivery.
This involves managing the client’s complete device lifecycle.
At the start of the cycle Insight procured a variety of devices ranging from legacy Toshibas to Dell two-in-one 7210 laptops, and stored them in its warehouses.
The Insight integration team then loaded the client’s specialist software onto the devices and bundled them with the client’s study materials, which are specific to each of the medical trials.
Insight prepared each device for use straight out of the box, before packing and sending them out to the client’s sites. Once the laptops were safely on site, medical professionals can collect the clinical trial data and enter it onto the devices.
When each clinical study has been completed, Insight arranges for the return of old devices and accessories to its warehouses.
As the devices contain sensitive patient data, it is important to hold them securely before they are wiped clean and returned into storage. Insight carries out the data wipe according to a workflow it has agreed with the client.
Once all the patient data has been permanently removed, Insight either grades the devices to be reused, or sends them for secure disposal so a certificate of destruction can be provided to the client.
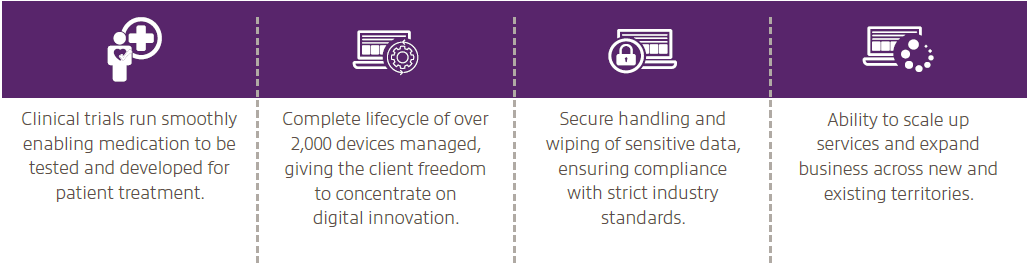
In the first twelve months of the project, Insight has managed more than 850 shipments of devices and study materials to sites across Europe.
The Benefits
- The ability to expand business globally.
- The capacity to take on larger contracts and service new clinical studies.
- The option to scale up existing services quickly and easily.
- Full device lifecycle managed from procurement, storage, installation and delivery, through to return and disposal.
- Freedom to focus on developing innovative technology without needing to spend time on managing hardware.
- No need to increase expenditure on managing operations such as storage and delivery of devices.
- Peace of mind that all sensitive data is securely wiped from the used devices.
- Certificate of Destruction issued for all devices sent for disposal, according to industry requirements.
The Results Highlights
Quick Overview
Client:
A leader in clinical trial software development.
Size:
A company of 200+ employees forming part of a healthcare group employing almost 2,500 people across the world.
Challenge:
To meet the demands of their global pharmaceutical customers in supplying a standardised service in challenging geographies.
Insight Solution:
End-user device lifecycle management and supply chain optimisation.